What are the hydrogen storage technologies? (II) - Physically based storage (gas or liquid)
Hydrogen storage methods used by industry and academia can be divided into two types:
Physics-based storage (gaseous or liquid)
Materials-based storage (hydrogen interacting with storage materials)
In materials-based storage, hydrogen is stored in three different media: metal hydrides (solid media), liquid hydrogen carriers, and material surface storage.
2.1 Metal hydride storage
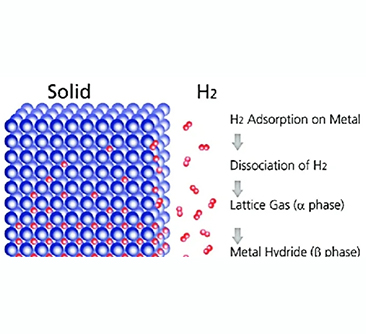
Molecular hydrogen adsorbed to the metal surface in a metal hydride storage system, and then the element (H) is introduced into the metal lattice via heat output and released along with the heat input. Compared with traditional physical-based storage, metal hydrides have many advantages, such as high volume density, safety, low operating temperature and pressure, etc. The bulk density of most hydrides is well above 80 kg/m3.
Metal hydride is a compound composed of certain metal elements and hydrogen element. Such compounds have active chemical properties and small reserves, but they have high use value. Metal hydride is when filling hydrogen, exothermic; when released hydrogen, endothermic; this heat release-endotherm cycle can be used to store and transmit heat
2.2 Liquid organic hydrogen carrier
The principle of liquid organic hydrogen storage technology (LOHC for short) is to use a pair of reversible reactions between hydrogen storage agents such as olefins, alkynes or aromatic hydrocarbons and hydrogen to achieve hydrogenation and dehydrogenation. The mass hydrogen storage density is between 5% and 10%, has a large hydrogen storage capacity, and the hydrogen storage material is liquid organic matter, which can be transported at normal temperature and pressure, which is convenient and safe. LOHC can chemically store hydrogen at high density under nominal environmental conditions. when filling hydrogen, exothermic; when released hydrogen, endothermic.
2.3 Surface storage systems (adsorbents)
Hydrogen can also be reversibly stored as sorbate by adsorption, via van der Waals forces, in materials with very specific surface areas. Some adsorbent materials include metal-organic frameworks (MOFs), zeolites, carbon nanotubes, etc. The required operating temperatures are lower and the pressures with this form of storage are higher. Surface storage systems also have low volumetric density.