What Are You Looking For?
PEM Hydrogen Production | Electrocatalysts for Membrane Electrodes
MEA (membrane electrode assembly) is a key part for proton exchange membrane water electrolysis (PEMWE) [also a key core part for PEM fuel cells], which includes a proton exchange membrane, a catalyst layer (CL) and a gas diffusion layer (GDL). As the place where the electrode reaction occurs, the structure of CL and GDL has a great influence on the transfer of reactants and products (gas-liquid two-phase flow), resistance, the movement of protons and electrons, and other related processes of the electrode reaction.The catalyst layer is the part that supports the catalyst, and the cathode catalyst layer and the anode catalyst respectively constitute the hydrogen evolution electrode and the oxygen evolution electrode. The catalytic substance of the cathode catalyst layer is usually platinum, usually platinum nanoparticles supported on porous nanocarbon. The catalytic substance of the anode catalyst layer is usually iridium or its oxide (such as iridium dioxide). The proton exchange membrane is between two electrodes embedded with a catalyst. The proton exchange membrane insulates the two electrodes from each other. The two electrodes constitute the anode and cathode respectively.
In PEM water electrolysis, carbon-supported platinum nanoparticles are used as electrocatalysts at the cathode [hydrogen evolution electrode] to promote HER (hydrogen evolution reaction). The hydrogen evolution reaction occurs at the negative electrode, and the hydrogen evolution reaction is as follows:
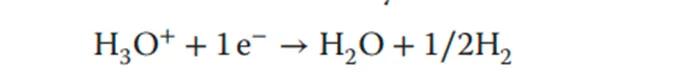
On the anode side, there is almost no supporting electron carrier available. Iridium (metal or oxide) is the most effective and stable OER catalyst in acidic media, and the anode reaction equation is as follows:
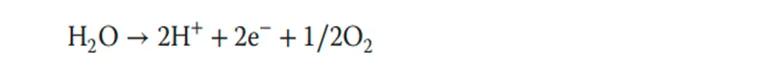
At present, the most widely concerned issue in the preparation of catalysts is the cost issue, because of the use of precious metals such as platinum and iridium. This is a key factor restricting the development of PEM hydrogen production and PEM fuel cells. The application of PEM hydrogen production needs to reduce the amount of platinum and iridium per unit area, optimize the membrane electrode preparation process and membrane structure, and ensure the stability of catalyst performance and structure.