What Are You Looking For?
Alkaline electrolysis water hydrogen production technology principle and market status
Alkaline electrolysis water technology (ALK): Usually potassium hydroxide (KOH) solution is used as the electrolyte, a porous membrane is used as the diaphragm, and use non-noble metal nickel-based catalyst. The advantage of this technology is that it is mature and low-priced. It is the main water electrolysis technology.
The structure of the electrolyzer: Alkaline electrolyzers are mainly composed of polar plates (bipolar plates + pole frames), catalytic electrodes, diaphragms, seals and other components.
Catalytic electrodes: most of the electrodes currently used in alkaline electrolytic cells are nickel-based materials. They are mostly made of pure nickel mesh and nickel foam as the base material, and are coated with catalysts using processes such as spraying, roller coating, and electroless plating to improve electrolysis efficiency. Most of the catalysts are Nickel-based catalysts represented by Raney Nickel or precious metal catalysts, etc.
Diaphragms: Initially, asbestos separator materials were mainly used, but the swelling of asbestos in alkaline electrolyte and the harm of asbestos to the people results it gradually eliminated. At present, the separator widely used in the industry is a new composite separator based on polyphenylene sulfide (PPS). PPS, as the base, can provide certain physical support. At the same time, PPS fabric has the characteristics of excellent heat resistance, high mechanical strength and excellent electrical properties. However, the hydrophilicity of PPS material is weak, which will cause the internal resistance of the electrolytic cell to be too large. Therefore, PPS is currently modified, such as coating polymer and zirconium oxide to form a composite separator to enhance its hydrophilicity.
Polar plate: It is the supporting component of the alkaline electrolyzer. Its function is to support the electrode and separator and conduct electricity.
Working principle: Alkaline electrolyzers usually use 30% potassium hydroxide solution (KOH) or 25% sodium hydroxide solution (NaOH) as the electrolyte. Under the action of direct current, water molecules undergo a hydrogen evolution reduction reaction at the cathode. Hydrogen gas and hydroxyl ions are generated. The hydroxide ions pass through the separator material under the action of the electric field, reach the anode, and lose electrons to generate oxygen and water.
The electrolytic water hydrogen production system mainly includes the electrolyzer and the BOP auxiliary system:
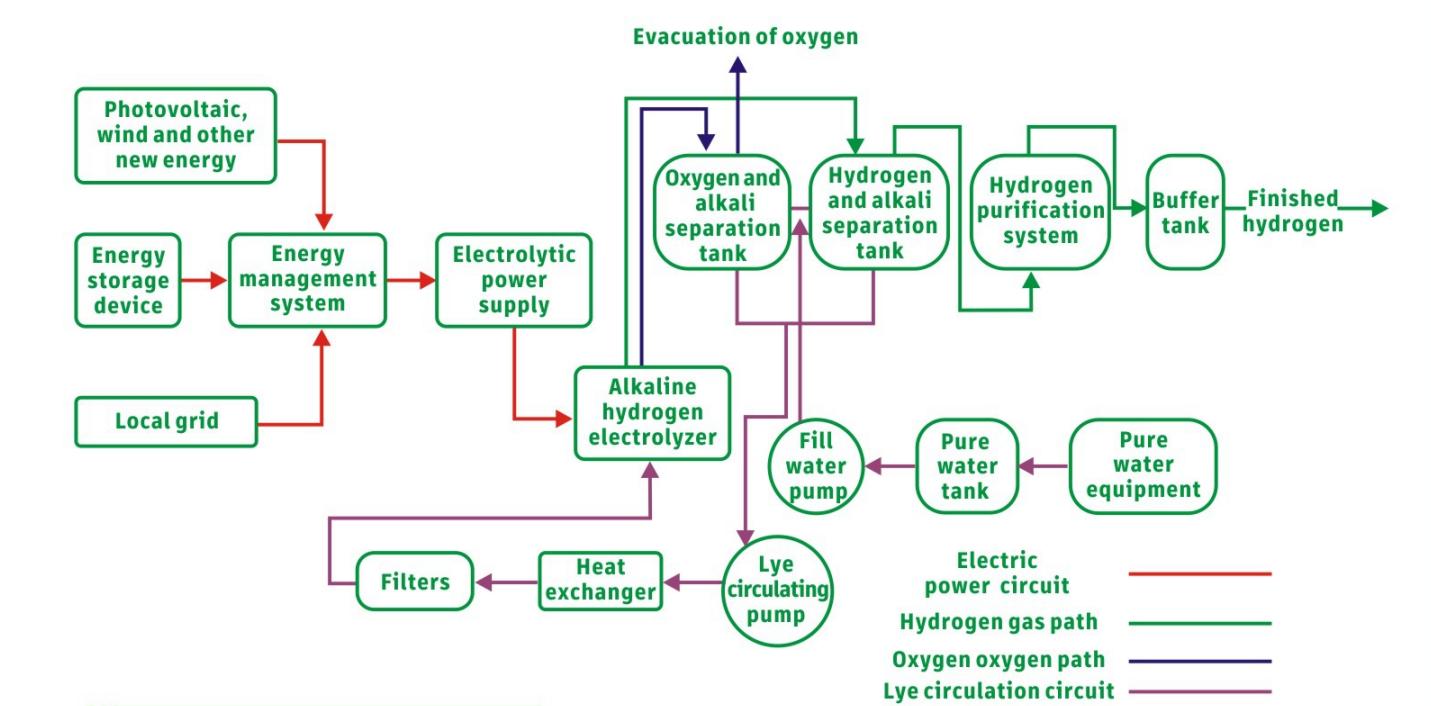
The BOP auxiliary system consists of power supply equipment (power supply, transformer, rectifier, etc.), gas-liquid separation & drying and purification equipment and other equipment.
Generally, the cost of an alkaline electrolysis water hydrogen production system is composed of: electrolyzer (50%), electrical equipment (15%), gas separation, drying and purification equipment (15%), and other equipment (20%).
In the electrolyzer, diaphragm and electrode assembly (57%), stack assembly & end plate (10%), bipolar plate (7%), small components (4%), structural layer (14 %), and the porous transmission layer (8%) is the main component of electrolysis.